Marine organisms are incredibly diverse and comprise an enormous number of species. Due to the ocean’s massive amount of biodiversity, many novel adaptations have evolved in marine organisms that are found in very few other environments. One of these amazing characteristics is symbiotic bioluminescence. Bioluminescence is common to a variety of marine organisms and can be used as camouflage, predator evasion, sensory confusion, and a hunting aid.
The bobtail squid however, has a unique situation in which it develops as a result of bacterial colonization in order to achieve its full bioluminescent capacity. The bobtail squid has a symbiotic relationship with the luminescent bacteria Vibrio fischeri, in which the bacteria gains a stable living environment and the squid develops and thrives in such a way that would be impossible without a symbiotic relationship. Due to its fascinating developmental history, and unique changes in both its light organ and circulatory system, the bobtail squid is now being used as a model for the study of symbiotic relationships.
Bioluminescence in marine organisms provides a host of different benefits and fills a number of different roles. Especially in the case of smaller organisms, bioluminescence is used as a form of camouflage, one such form is known as counter illumination. Counter illumination is meant to mimic the filtering of light from either the sky or the water’s surface, and thus obscure the ventral silhouette of the organism. This form of luminescence is common to squid and other cephalopods, some crustaceans and some fish.
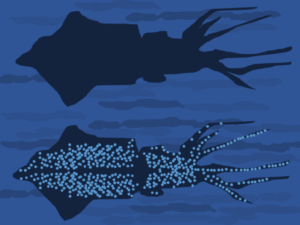
Counter illumination is achieved either by pigmentation only from the organism or through a symbiotic relationship with florescent bacteria (Lindgren, 2012). Another way in which luminescence is used in organisms is to hide from prey; in a recent study, counter illumination was discovered in 21 unique sharks that appear to emit a near constant luminescence (Claes, 2013). The sharks are not often subject to predation, so it is assumed that the luminescence is in an attempt to mimic the prey instead of hide from predators (Claes, 2013). In other situations, such as in lantern fish, only a single structure is bioluminescent and is used to lure in prey instead of hide from predators. Finally, bioluminescence can be used as a distraction, like in the case of Acanthephyra purpurea, a deep-sea shrimp, which vomits a bioluminescent substance to distract predators while it escapes.

Bobtail squid (member of the mollusk order Sepiolida) utilize luminesce in order to hide from predators and blend their silhouette into the water above it. In recent years, these squid have been used by biologists as a model to study symbiotic luminescence.

The developmental changes bobtail squid undergo when colonized by the bacteria are both dramatic and accessible for biologists to study. The bobtail squid, Euprymna scolopes, hatches as a fully formed squid. They have no larval stage. However, there’s a vestige of the ciliated structures seen in other mollusks. (Cilia are like tiny hairs on the surface of individual cells.) These ciliated cells cover the surface of a pair of “appendages” in the undeveloped light organ (Koropatnick, 2014). After hatching, the bobtail squid has no association with any bacteria, and is not capable of luminescence. The light organ can only complete its development once it is colonized by a specific species of bacteria, Vibrio fischeri.
Once V. fischeri is obtained from the surrounding seawater, the bacteria become suspended in mucus and migrate to the light organ. The cells smell their way! They follow a trail of chemical cues left by the squid’s cells (Koropatnick, 2014). Approximately 12 hours after hatching, the light organ is fully colonized. Once the ciliated appendages have brought in enough bacteria they regress. They’ve served their purpose and are no longer necessary. The bacteria release proteins that cause the appendages degrade, transforming the structure into a fully formed light organ. This changer involves widespread cell death and the loss of cilia (Koropatnick, 2014). Only cells of the ciliated appendages however are susceptible to this destructive signal. By 18 hours after hatching the entire process is nearly completed!
After the light organ is formed, the symbiosis is regulated by daily venting. In addition to forming the light organ itself, the bobtail squid must also produce a ventilation system in order to control the number of bacteria. Each morning around dawn, one species, Euprymna scolopes, expels 95% of the bacteria currently living inside its light organ. This process accomplishes several goals. First, bioluminescence is unnecessary during the daytime for animals near the ocean surface, like bobtail squid. It is also possible, that this expulsion controls the extent to which the squid adapts to varying light conditions, and matches its level of luminescence to its surroundings (Jones, 2004). Although the squid gains an advantage by venting, the bacteria don’t appear to benefit or suffer from the expulsion. They are able to recolonize the light organ again before night time. However venting does seem to provide bacteria that can be used by hatchling bobtail individuals nearby for the development of their light organs (Jones, 2004).
While V. fischeri in open water can still fluoresce, they are about 100 times brighter in symbiosis with a bobtail squid. One reason for this is that the proteins that produce light require oxygen to work. Because animals move around, their cells require oxygen too, and animals have circulatory systems that move oxygen around very well. In the bobtail squid, blood picks up oxygen in the gills and then travels through the circulatory system. It passes into tiny blood vessels in the light organ, where oxygen can then be taken up by the symbiotic bacteria (Kremer, 2014), which contributes to the overall luminescence of the squid (Dunlap, 2014). In this way, the squid shares about 10% of its acquired oxygen with the bacteria.
In other cephalopods (the mollusk group that includes squid and octopus), the light organ is not necessarily the same as in the bobtail squid. In the octopus Japetella diaphana colonization occurs in a luminous mouth ring. The dramatically named “glowing sucker octopus” has a ‘light organ’ that is actually the sucking muscle. Another octopus, Cistopus, collects luminescent bacteria within its head and cranial spaces (Naguit, 2014).

As dramatic as the developmental processes of the symbiotic relationship in the bobtail squid, other organisms also have elaborate developmental events to achieve symbiosis. The silver ponyfish Nuchequula nuchalis requires colonization of its light organ for full development of other internal organs such as the gallbladder and stomach (Dunlap, 2008).
Although the changes induced in the host organism are quite varied, the benefits to the bacteria, which colonize the organisms, are generally very similar. This is most likely due to the fact that there is not a large variety of bioluminescent bacteria species that act in bioluminescent symbiosis. The most common symbiotic bacteria come from only two genera, Vibrio and Photobacterium. Each species colonizes a host and provides them with luminescence, and gains a few advantages in return. The simplest result of the symbiotic relationship is a place to live; if the bacteria are forced to live in open water and lack a stable environment, life can be hard with less available food and lots of predators in the plankton. Therefore, symbiotic luminescence does appear to be beneficial to both the host and symbiont.
Symbiotic luminescence is not a heavily studied subject, but is nevertheless fascinating. The use of the bobtail squid as a model species allows scientists to study the ways in which developmental steps in an organism’s life can be triggered by a symbiotic relationship. However, there may be many more organisms that develop in unique ways that are as of yet undiscovered.
Citations
Claes, J. M., Nilsson, D., Straube, N., Collin, S. P., Mallefet, J. Iso-luminance counter-illumination drove bioluminescent shark radiation. Scientific Reports, 4 (2014)
Dunlap, P. Biochemistry and genetics of bacterial bioluminescence (2014) Advances in Biochemical Engineering/Biotechnology, 144, pp. 37-64.
Dunlap, P.V., Davis, K.M., Tomiyama, S., Fujino, M., Fukui, A. Developmental and microbiological analysis of the inception of bioluminescent symbiosis in the marine fish Nuchequula nuchalis (Perciformes: Leiognathidae) (2008) Applied and Environmental Microbiology, 74 (24), pp. 7471-7481
Dunlap, P.V., Takami, M., Wakatsuki, S., Hendry, T.A., Sezaki, K., Fukui, A. Inception of bioluminescent symbiosis in early developmental stages of the deep-sea fish, Coelorinchus kishinouyei (Gadiformes: Macrouridae). (21014) Ichthyological Research, 61 (1) pp. 59-67.
Jones B.W., Nishiguci M.K., Counter illumination in the Hawaiian bobtail squid, Euprymna scolopes Berry (Mollusca: Cephalopoda). Marine Biology, 144 (2004), pp. 1151–1155
Koropatnick, T.A., Goodson, M.S. Heath-Heckman, E.A. McFall-Ngai, M. Identifying the cellular mechanisms of symbiont-induced epithelial morphogenesis in the squid-Vibrio association. Biological Bulletin 226 (2014), pp. 56–68
Kremer N, Schwartzman J, Augustin R, Zhou L, Ruby EG, Hourdez S, McFall-Ngai MJ. The dual nature of haemocyanin in the establishment and persistence of the squid-vibrio symbiosis. Proc Biol ScI (2014)
Lindgren A. R., Pankey M.S., Hochberg F. G., Oakley T. H., A Multi-Gene Phylogeny of Cephalopoda Supports Convergent Morphological Evolution in Association with Multiple Habitat Shifts in the Marine Environment. (2012) BMC Evolutionary Biology, 12 (129).
Naguit, M.A.A., Plata, K.C., Abisado, R.G., Calugay, R.J. Evidence of bacterial bioluminescence in a Philippine squid and octopus hosts. (2014) AACL Bioflux, 7 (6), pp. 497-507




